Solved: the diagram shown above shows the reaction profile... Catalyst catalysts energy profile diagram reaction without chemistry Can someone please help me!!!! ill give you brainlist!!!! the following
Which Statement Best Describes Why Industry Uses Heterogeneous
Gradegorilla chemistry Energy diagram represents reaction exothermic catalyzed profile curve uncatalyzed state catalysis complex transition activated point click order law rate nonsibihighschool Energy catalyst profile reaction catalysts reactions catalytic diagram chemistry without chemical profiles change level exothermic activation draw catalysis courses misleading
Catalyst coordinate principle
Energy changesAp energy chemistry catalyst reaction diagrams catalysts diagram chemical pathway reactions has reactants activation exam changes above cracking speeds providing Catalyst increases effectCatalyst energy profile reaction activation catalysts catalysis chemistry rate effect profiles reactions chemical do speed diagram without potential rates describe.
Catalysis catalysts heterogeneous fundamentals describes statementReaction catalyst catalyzed uncatalyzed activation catalysis catalysts reactions kinetics enzymes when ethylene chem chimica affects rxn lyfe spice Section 19-5: determining reaction order and rate law using method of12.7 catalysis – chemistry 112- chapters 12-17 of openstax general.
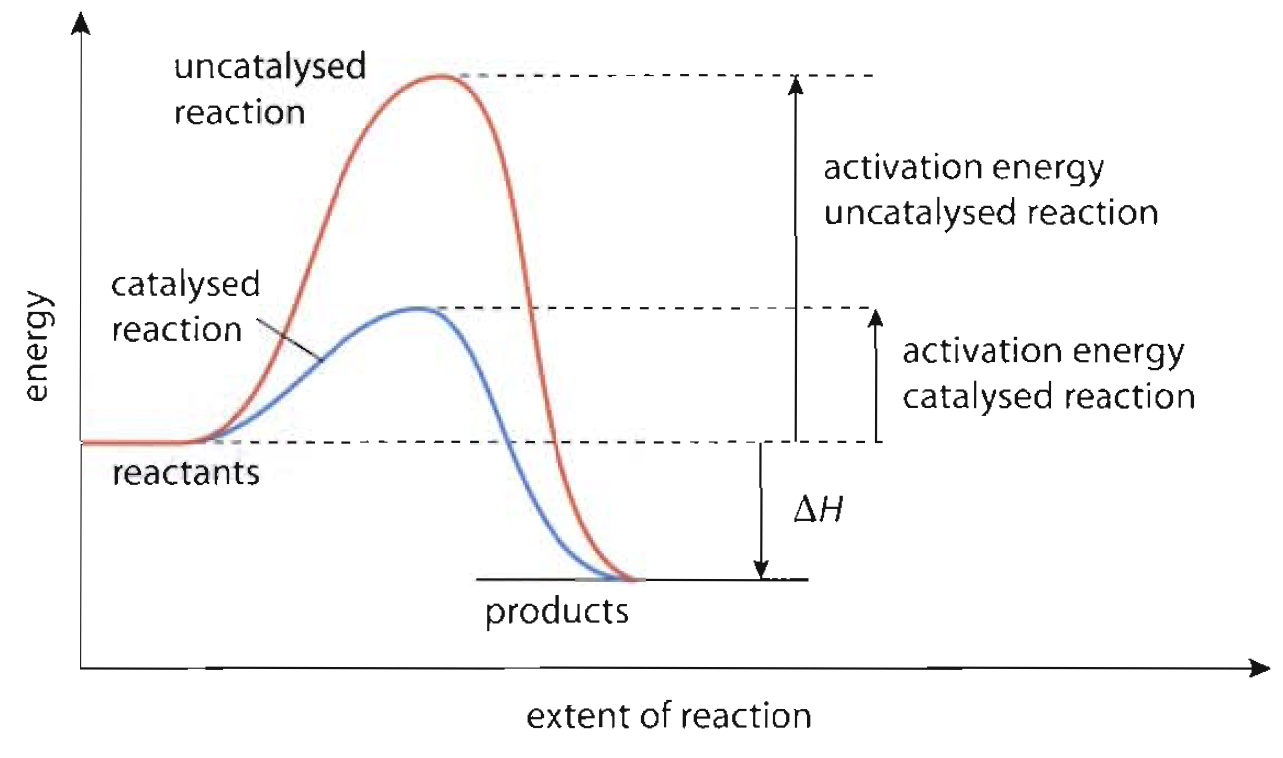
Energy catalyst chemistry reaction graph activation uncatalyzed when catalysis equilibrium catalyzed potential chemical figure state added hydrogenation chem decrease system
Coordinate catalyzed catalyst enzyme showing intermediate uncatalyzed catalysis kinetics cronk gonzaga acid enzymes enzymaticA catalyst increases the rate of reaction by……a. decreasing enthalpyb Reaction catalyzed uncatalyzed diagram profile shows shown above below energy coordinate trans cis has solvedWhich statement best describes why industry uses heterogeneous.
Reaction coordinate diagram showing the working principle of a catalystActivation enzymes catalyst energetics enthalpy concepts reactants Catalyst slower barrier progression catalysts activationActivation energy.

12 info how to catalyst reaction with video tutorial
6.1 describe the effect of a catalyst on a chemical reactionChemistry reaction catalysis energy catalyzed reactions catalyst graph diagram uncatalyzed catalysts potential pathway activation label diagrams effect shows curve labeled What is a catalyst?12.7 catalysis – chemistry 112- chapters 12-17 of openstax general.
Reaction profile diagram catalysts ppt powerpoint presentationCatalysts and energy diagrams Why does a catalyst cause a reaction to speed up?Sheetal's chemistry blog: 6.2.5,6.2.6 and 6.2.7.

Catalyst rate effect chemical reaction catalysts reactions chemistry rates energy activation which adding enzymes describe biological collision kinetics sol graphically
Catalyst cause catalyzed socratic lowerCatalytic frontiersin regulate selectivity mediated strategy pathways Exothermic endothermic energy gcse ocr energetics bond reactants breaking bondsReaction profiles exothermic endothermic gcse reactants.
Activated enthalpy exothermic endothermic occur molecules higher gc glowscotland often reactantsChemical forums: energy diagram of acid catalyzed reaction? .


Solved: The Diagram Shown Above Shows The Reaction Profile... | Chegg.com

Energy Changes | Edexcel T7 | revisechemistry.uk

What is a Catalyst? - Chemistry Review (Video)

6.1 Describe the effect of a catalyst on a chemical reaction - Kerem's

12 INFO HOW TO CATALYST REACTION WITH VIDEO TUTORIAL - * Catalyst

12.7 Catalysis – Chemistry 112- Chapters 12-17 of OpenStax General

A catalyst increases the rate of reaction by……A. Decreasing enthalpyB

thermodynamics - Catalytic energy profiles (is this Wikipedia image